Senza Spinal Cord Stimulation System – P130022/S039
Senza Spinal Cord Stimulation System – P130022/S039
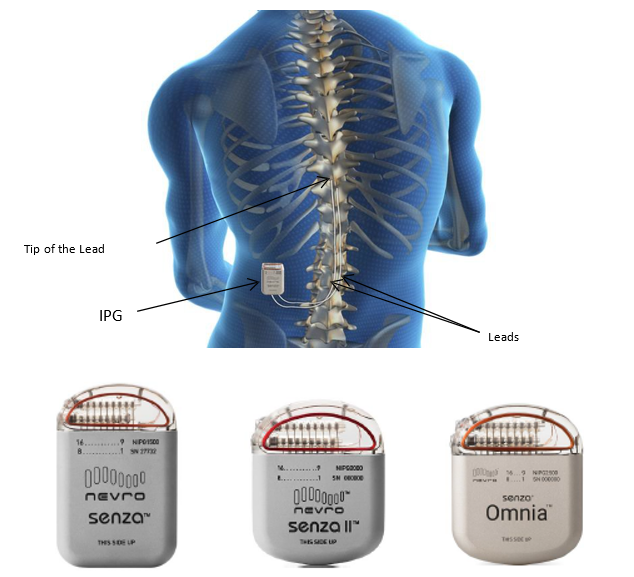
The Senza, Senza II, and Senza Omnia are implanted, rechargeable Spinal Cord Stimulation systems to treat chronic pain in a patient’s trunk or limbs that is difficult to manage.

Spinal Cord Stimulator Procedure, Recovery, & Restrictions

FDA Approves First Smart Spinal Cord Stimulation Trial System

Spinal cord stimulation

FDA Approves Spinal Cord Stimulation Therapy for Painful Diabetic

FDA approves Nevro HFX iQ spinal cord stimulation

Senza Spinal Cord Stimulation System – P130022/S039
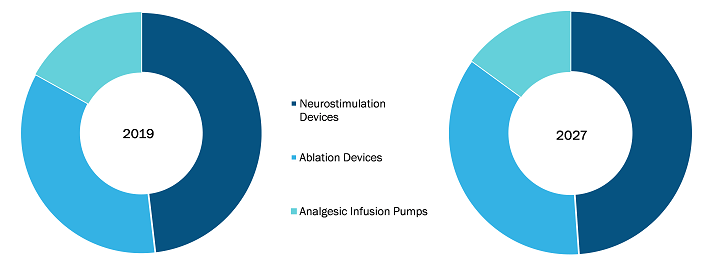
Pain Management Devices Market Growth Report
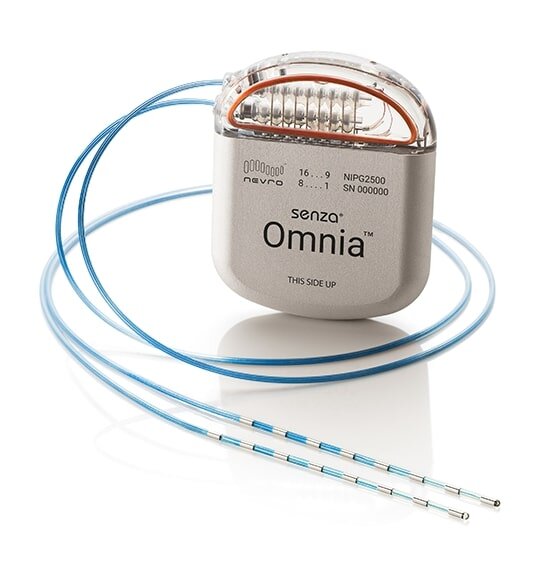
FDA Approves Spinal Cord Stimulator for Diabetic Neuropathy — Pain
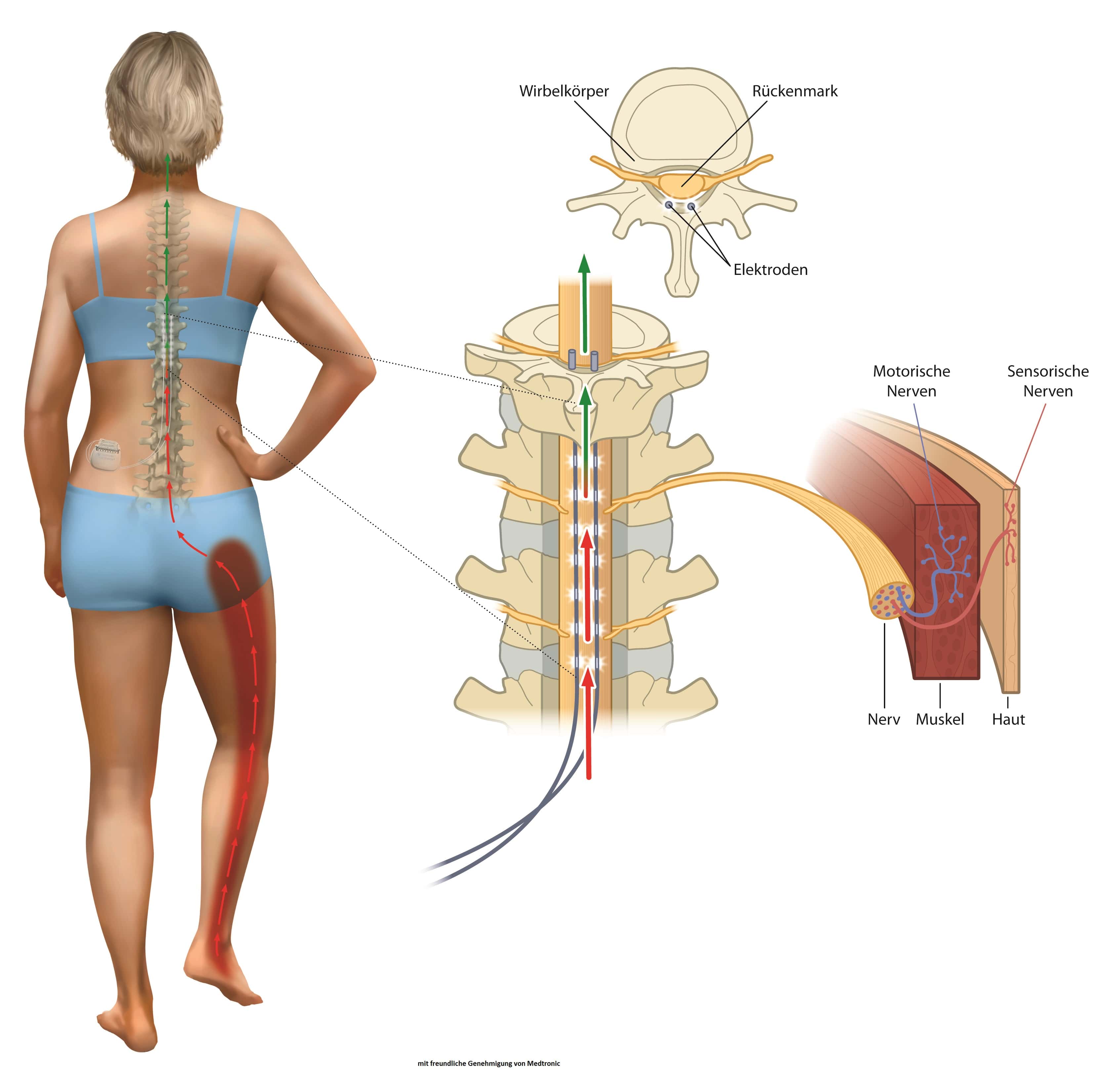
Spinal Cord Stimulation - Pain pacemaker
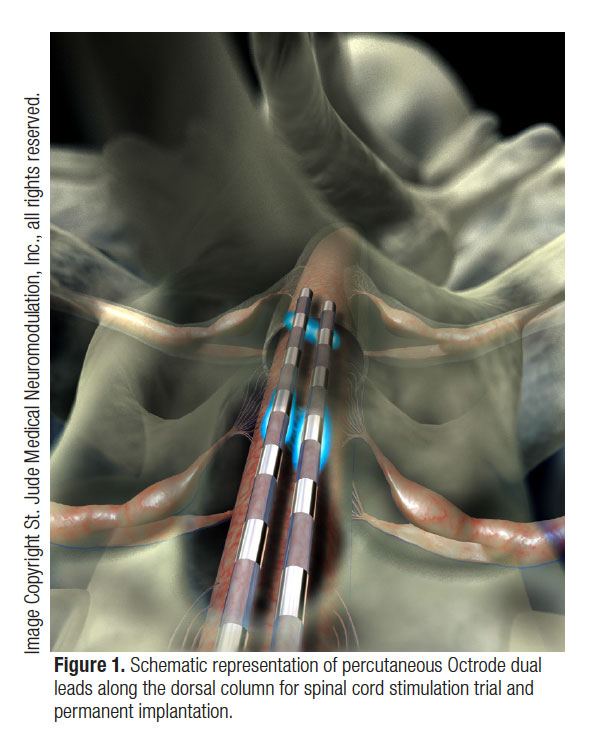
Spinal Cord Stimulation: Fundamentals